All rights reserved:Jiangxi Biological Products Research Institute Co., Ltd.
�:
Production system
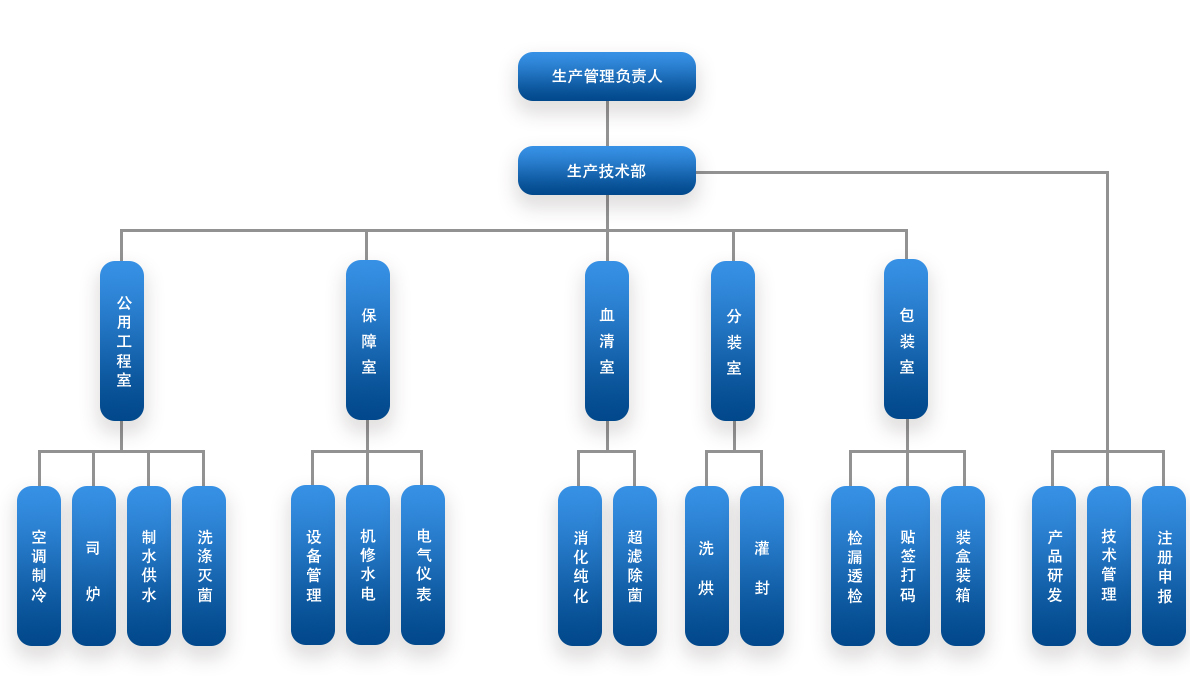
1. Ensure that the production and packaging of the drug is operated in accordance with the approved process specifications and operating procedures and relevant records to ensure that the drug meets the specified quality standards and meets the requirements for drug production license and registration approval.
2. Responsible for the formulation and implementation of the company's annual, monthly and weekly production plans and production instructions;
3. Reasonably allocate personnel in various positions in the department, organize production according to GMP requirements, and ensure that production personnel operate in strict accordance with the "production process regulations" and "post operation procedures" to ensure normal production and ensure product quality;
4. Responsible for the compilation, training and implementation of production management, health management, equipment management and other documents;
5. Responsible for the rational allocation of public works operators according to the production plan and batch production instructions to ensure that various media (clean air, steam, process water, oxygen, nitrogen, compressed air, hydropower, liquefied petroleum gas, cold and heat, etc.) meet the process quality. Requirements and meet the production needs of the product;
6. Cooperate with relevant departments on the verification of plant, facilities, equipment, air purification system, process water system and other products directly related to product quality, and inspect the routine maintenance and safety management of plant facilities and equipment to ensure the plant, facilities, Normal operation of equipment, etc.
7. Responsible for GMP training, professional knowledge training, job operation training and assessment work for production personnel.
8. Responsible for the on-site management of the workshop, guiding production personnel to use the equipment correctly, ensuring good production order and safety and sanitation;
9. Assist in the regular calibration of instruments and meters and the regular inspection of pressure vessels;
10. Responsible for guiding and checking the filling of production records, and doing a good job in statistics, analysis and management of production technology economic indicators;
11. Assist the Quality Department in carrying out technological innovation work;
12. Participate in the audit of the supplier;
13. The Collaborative Quality Department determines the approval and supervision of the entrusted (processing or packaging) party.


